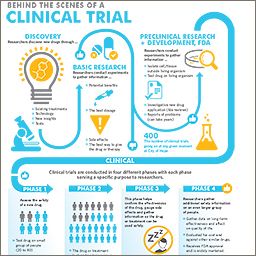
Clinical trials play a crucial role in advancing medical knowledge and bringing new treatments to patients. However, managing and conducting clinical trials can be a complex and time-consuming process. EMR software has emerged as a powerful tool to streamline and optimize various aspects of clinical trials.
Efficient Participant Recruitment and Management
EMR software enables researchers to efficiently identify and recruit eligible participants for clinical trials. By leveraging the comprehensive patient data stored in EMR systems, researchers can quickly identify potential candidates based on specific criteria, such as age, medical conditions, and demographics. This streamlines the recruitment process, ensuring that trials can enroll participants more efficiently.
Once participants are enrolled, EMR software simplifies the management of trial-related data. It allows for easy tracking of participant progress, documentation of adverse events, and efficient communication between research teams and participants. EMR software streamlines the administrative tasks associated with participant management, ensuring smoother trial operations.
Seamless Data Collection and Integration for Clinical Trials
EMR software eliminates the need for manual data entry and paperwork, enhancing the accuracy and efficiency of data collection during clinical trials. Researchers can directly capture data from patient encounters, laboratory results, and other relevant sources, reducing the chances of transcription errors and ensuring data integrity.
Moreover, EMR software facilitates the integration of data from multiple sources, such as medical imaging systems or laboratory information systems. This integration enables researchers to gather comprehensive and real-time data, enhancing the depth and quality of information available for analysis.
Enhanced Collaboration and Communication
Effective collaboration and communication among research teams are crucial for successful clinical trials. EMR software provides a centralized platform for research teams to access and share trial-related information securely. It allows for real-time collaboration, ensuring that all team members have access to the latest data, study protocols, and updates.
Furthermore, EMR software enables seamless communication between researchers, study coordinators, and other stakeholders involved in the clinical trial. This streamlines the exchange of information, enhances decision-making processes, and facilitates timely interventions if necessary.
Improved Data Analysis and Reporting for Clinical Trials
EMR software simplifies the process of data analysis and reporting for clinical trials. Researchers can leverage the advanced reporting capabilities within EMR systems to generate real-time reports on trial progress, participant demographics, adverse events, and outcomes. This empowers researchers to monitor trial efficacy, identify potential safety concerns, and make data-driven decisions throughout the trial.
In addition, EMR software supports the integration of electronic Case Report Forms (eCRFs) into the trial workflow. This eliminates the need for manual data entry into separate databases, reducing redundancy and minimizing errors in data analysis.